X-RAYS
X-rays are generated when free electrons give up some of their energy when they interact with the orbital electrons (k-shell)or nucleus of an atom (Brehmsstrahlung).
X-rays are generated when free electrons give up some of their energy when they interact with the orbital electrons (k-shell)or nucleus of an atom (Brehmsstrahlung).
REQUIREMENTS FOR PRODUCTION:
To generate X-rays, we must have three things. We need to have a source of electrons, a means of accelerating the electrons at high speeds, and a target material to receive the impact of the electrons and interact with them.So what elements emit X-ray lines?
The more protons an element has, more energetic its lines can be. Carbon atoms (6 protons each) can emit X rays. But carbon lines are at the low end of X rays. Many X-ray instruments cannot detect these photons. Of the common elements in the universe, iron (26 protons) and oxygen (8 protons) usually are the two most prominent sources of X-ray lines along with tungsten and copper.
X-RAY PRODUCTION:
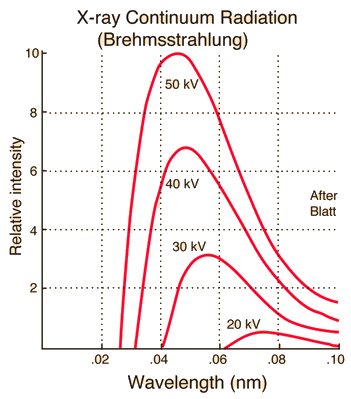
Bremsstrahlung X-rays
In an X-ray tube the electrons emitted from the cathode are accelerated towards the metal target anode by an accelerating voltage of typically 50 kV.
In the Bremsstrahlung process, a high speed electron traveling in a material is slowed or completely stopped by the forces of any atom it encounters.
If the electron is slowed down, it will exit the material with lesser energy.( The law of conservation of energy tells us that this energy cannot be lost and must be absorbed by the atom or converted to another form of energy). The energy used to slow the electron is excessive to the atom and the energy will be radiated as x-radiation of equal energy.
If the electron is completely stopped by the strong positive force of the nucleus, the radiated x-ray energy will have an energy equal to the total kinetic energy of the electron. This type of action occurs with very large and heavy nuclei materials.
K-SHELL EMISSION
The K-shell is the lowest energy state of an atom. An incoming electron can give a K-shell electron enough energy to knock it out of its energy state. About 0.1% of the electrons produce K-shell vacancies; most produce heat. Then, a tungsten electron of higher energy (from an outer shell) can fall into the K-shell. The energy lost by the falling electron shows up in an emitted x-ray photon. Meanwhile, higher energy electrons fall into the vacated energy state in the outer shell, and so on.
When outer-shell electrons drop into inner shells, they emit a quantized photon "characteristic" of the element. The energies of the characteristic X-rays produced are only very weakly dependent on the chemical structure in which the atom is bound, indicating that the non-bonding shells of atoms are the X-ray source.
K-shell emission produces higher-intensity x-rays than Bremsstrahlung, and the x-ray photon comes out at a single wavelength.
The X-ray photons produced by electrons that move from higher energy levels to k-shells, form the
K-SERIES.
If the electron moves from L-SHELL to K-SHELL,it gives rise to the first line of the K-SERIES which is "kα".
If the electron moves from M-SHELL to K-SHELL,it gives rise to the second line of the K-SERIES which is "kβ".
If the electron moves from N-SHELL to K-SHELL,it gives rise to the third line of the K-SERIES which is "kγ".
Similarly X-ray photons produced by movement of electrons from higher energy levels to L-shells constitute the L-SERIES and those into M-shells make up the M-SERIES.

.jpg)


No comments:
Post a Comment